MIGHTY-Fer (syr)
-
Chemical Name:
Iron (III) hydroxide polymaltose complex -
Therapeutic Category:
Gynecological drugs -
Pharmacologic Category:
Iron Supplement -
Pharmaceutical Form:
Syrup -
Composition:
Iron (III) hydroxide polymaltose complex eq. Iron 50mg/5ml
MIGHTY FER
Chewable Tablets - SyrupInjection (Ampoule)
Iron (III) Hydroxide Polymaltose Complex
1. PHARMACOLOGY:
MIGHTY-FER provides an ideal preparation for the treatment of all iron deficiency anemias due to its rapid absorption, its high rate of iron utilization, the effective Hb increase and its excellent degree of tolerability.
MIGHTY-FER is unlikely to cause tooth straining or to give rise to irritation of the intestinal mucosa. Until now interactions have not been observed. Since the iron is complex-bound, ionic interactions with foodstuff components (phytin, oxalates, tannic etc.) and concomitant administration of medicaments (tetracylines, antacids) are unlikely to occur.
In cases of overdosage neither intoxication nor iron overload have been reported to date because the iron in the active substance iron (III)-hydroxide polymaltose complex does not exist in the gastrointestinal tract as free iron and is not taken up by the organism by passive diffusion.
2. INDICATIONS:
* Tablets & Syrup:
Treatment of latent iron deficiency and iron deficiency anemia (manifest iron deficiency).
* I.M Injection:
All cases of iron deficiency in which rapid and reliable substitution of iron is required, more particularly for the following:
- Severe iron deficiency, e.g. after haemorrhages.
- Disturbances in iron resorption in the gastrointestinal tract.
- Marked incompatibility of oral iron preparations.
- Iron deficiency during pregnancy.
- Iron deficiency resistant to treatment, when the patient cannot be relied on to take the tablets, and contact between doctor and patient at irregular intervals.
3. CONTRA-INDICATIONS:
- Hypersensitivity to any of the constituents.
- Iron overload (e.g. hemochromatosis, hemosiderosis) or disturbances in iron utilization (e.g. load anemia, sidero-achrestic anemia, thalassemia) and anemia not caused by iron deficiency (e.g. hemolytic anemia).
- All cases of iron surfeit and disturbances in utilization of iron, Crohn’s disease, bronchial asthma, progressive chronic polyarthritis.
4. PRECAUTIONS:
- Patients who are under oral treatment should interrupt the oral treatment 24 hours before the first I.M injection.
- Regular monitoring of the Iron levels should be done to avoid overdosage.
- Pregnancy and nursing women: MIGHTY Fer could be used safely.
5. SIDE EFFECTS:
* Tablets & Syrup:
There are no side effects.
* I.M Injection:
- It may cause a darkness of the urine.
- It may cause a pigmentation at the site of injection.
6. DRUG -INTERACTIONS:
The oral form of the Iron should not be given together with the Intramuscular form (Lipothymia): due to the rapid liberation of the iron from its complex form and to the saturation of the siderophiline.
7. DOSAGE & ADMINISTRATION:
1) Mighty-Fer Chewable Tablets:
1-2 Chewable Tablets daily after meals, or as per physician instructions.
2) Mighty-Fer Syrup:
Adults: (100 mg) 2 Teaspoonfuls (10 ml), 2-3 times daily after meals.
Children: (50 mg) 1 Teaspoonful (5 ml), 1-2 times daily after meals.
Infants: (25 mg) ½ Teaspoonful (2.5 ml), 1-2 times daily after meals.
Newborn: (2.5 mg Fe) Per kg body-weight daily divided in many drinks.
Mighty-Fer may be mixed with fruit, or vegetable juices or other liquids.
Mighty-Fer should be taken during or immediately after meals.
Best results are obtained by adequate dosage and regular administration.
The therapy should be continued for at least 1 to 2 months.
3) Mighty-Fer I.M Injection:
Dosage: Normal daily dose for adults is 1 ampoule (100 mg) per day.
Children are given correspondingly less, depending on their age.
Daily Maximum Doses:
Children Up to 5 kg: 0.5 ml (¼ Ampoule)
Children between 5-10 kg: 1.0 ml (½ Ampoule)
Adults: 4.0 ml (2 Ampoules)
* Duration of Treatment:
One injection each 3 to 7 days.
The intramuscular injection should be slow and deep.
Technique of Injection:
The technique of injection is of crucial importance.
The wrong method may result in pain and discoloration of the skin.
The following method of ventro-gluteal injection according to HOCHSTETTER is recommended instead of the normal method of injection in the top outer quadrant of the gluteus maximus muscle.
a) Length of needle at least 5-6 cm. The lumen of the needles should not be too wide.
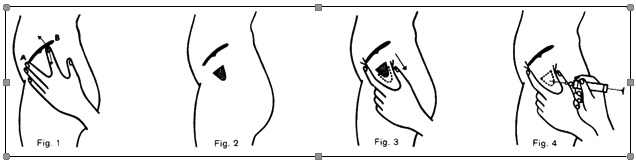
b) According to HOCHSTETTER, the site of injection is determined as follows (see Fig.1):
First point A is found, corresponding to the ventral iliac spine. If the patient lies on the right side, for instance, the middle finger of the left hand is placed on point A. The index finger is extended away from the middle finger, so that it comes to lie below the iliac crest, at point B. The triangle lying between the proximal phalanges of the middle and index fingers represents the side of injection. This is disinfected in the usual way (Fig. 2).
c) Before the needle is inserted, the skin over the site of injection is pulled down, about 2 cm (Fig. 3), to give an S-shaped puncture channel.
This prevents the injected solution from running back into the subcutaneous tissues and discoloring the skin.
d) The needle is introduced more or less vertically to the skin surface, angled to point towards the iliac crest rather than the hip joint (Fig. 4).
e) After the injection, the needle is slowly withdrawn and pressure from a finger applied beside the puncture site. This pressure is maintained for about one minute.
f) The patient should move after the injection.
8. PACKAGING & COMPOSITION:
- MIGHTY FER - Chewable Tablets: A pack of 20 or 24 chewable tablets. Each chewable tablet contains Iron (III) Hydroxide Polymaltose Complex equivalent to 100 mg Iron.
- MIGHTY FER - Syrup: A pack of glass bottle of 100 mL. Each 5 mL of syrup contains Iron (III) Hydroxide Polymaltose Complex equivalent to 50 mg Iron.
- MIGHTY FER - Injection: A pack of 5 ampoules of 2 mL. Each ampoule (2 mL) contains Iron (III) Hydroxide Polymaltose Complex equivalent to 100 mg Iron
9. STORAGE CONDITIONS:
Store MIGHTY FER – Tablets, Syrup and Injection at temperature between (20-25)°C.









